Metal and Mineral Toxicity Presenting as Neurological Complaints
Metal and Mineral Toxicity Presenting as Neurological Complaints
Dan Carter NDThe connection between toxic metals and neurological symptoms is well established, but it is not as well known that beneficial minerals in too high amounts can also result in toxicity and distinctive neurological syndromes. For simplicity, the term “mineral” will be used in the general discussion, whereas the specific term (i.e., “lead”) will be used when describing particular attributes of the mineral.
The minerals lead, mercury and manganese all can lead to debilitating mental, neurologic and physical symptoms, and while lead and mercury are toxic in any amount and have no nutritional value, manganese is a beneficial mineral. Manganese functions as part of the metaloenzymes and as an enzyme activator. Carbohydrate synthesis requires pyruvate carboxylase, a manganese-containing enzyme, and manganese superoxide dismutase catalyzes the simultaneous oxidation and reduction (disproportionation) of superoxide to hydrogen peroxide and water. Manganese is also required for ATP reactions, glutamine synthetase and the glycosyltransferases (Ziegler and Filer, 1996). Three cases demonstrate the diverse symptomatology and similarities of treatment.
Lead
Historical Perspective
The lead normally found in the earth’s crust is basically immobile and non-toxic. Once lead is mined and integrated into manufactured products and spread throughout the environment, it is highly toxic. Lead is the most widely dispersed toxic metal in the world, has a long-term environmental persistence and is always toxic if ingested or absorbed.
The toxicity of lead was documented by 2000 BC and its widespread use caused chronic plumbism in many societies historically. The Greeks reported the colic and anemia caused by lead poisoning in 250 BC. Hippocrates linked gout with food and wine but failed to realize that gout was caused by lead poisoning during his time (450-380 BC). Throughout the Roman period, gout was common in the upper classes and was thought to be due to high lead exposure (Needleman, 1999).
Pathophysiology
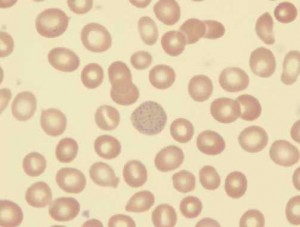
Lead blocks many enzyme systems. As with most heavy metals, any molecule containing sulfhydryl groups is susceptible to inhibition. The best known effect is on the interference with heme synthesis. Lead interferes with both aminolevulinic acid and the insertion of iron into the protoporphyrin molecule; this results in decreased heme production. Heme is essential for hemoglobin production, so cellular oxidation is affected. Stained blood smears commonly exhibit coarse basophilic stippling in the red cells (see Image 1).
The effects of lead poisoning on the brain include delayed or reversed development, permanent learning disabilities, seizures, coma and death with severe toxicity.
Lead is excreted by the kidneys, but the elimination rate varies depending on the route of absorption (e.g., skin, lungs, GI).
Death from lead encephalopathy was often seen in urban areas during the 1960s, but is rare today (Marcus, 2007).
Symptoms of Lead Toxicity
The human body does not use lead but absorbs and stores it as if it were calcium or phosphorus. Adults excrete lead much better than children do. Adults can excrete approximately 99% of the lead they absorb within a few weeks, whereas children will excrete approximately 32%.
With chronic exposure, lead is stored in organs and tissues, especially bones and teeth.
Common symptoms of chronic overexposure to lead include anorexia, metallic taste, anxiety, constipation, nausea, pallor, fatigue, weakness, insomnia, headache, irritability, muscle and joint pain, fine tremors, numbness, dizziness, hyperactivity and colic. In lead colic there may be severe abdominal pain (State of WA Dept. of Ecology, n.d).
Mercury
Historical Perspective
Elemental mercury was known to the ancient Greeks, Romans, Chinese and Hindus (Sloane, 2005).
Qin Shihuang, the first emperor of China, wanted a medicine that could make him immortal. His doctors prescribed a medicine that contained mercury, and it was this mercury that poisoned and eventually killed him (Chinaculture.org, 2003).
By the 1800s, mercuric nitrate was used to soften and form fur for hats. The exposure of workers led to a classic toxic syndrome, and the phrase “mad as a hatter” may have been used to describe it. In Danbury, Conn., a center of hatmaking, the effects of mercury toxicity were called the Danbury Shakes. It was not until 1941 that the use of mercury nitrate in hatmaking was banned in the U.S. (Chem Info Net, n.d).
Mercury comprises 43% to 54% of the ingredients in dental amalgam fillings; most of the remainder is silver. In 2002, the FDA published a proposed rule to classify dental amalgam as a class II device with special controls. Class II devices are those for which general controls alone are insufficient to assure safety and effectiveness. Canada, France and Sweden recommend that amalgam be avoided in pregnant women (US FDA, Q&A on Dental Amalgam, 2008). In Norway and Sweden, new dental amalgam fillings were banned as of Jan. 1. Denmark announced a similar ban that went into effect April 1 (Hiscott, 2008).
Since the 1930s, many multiple-dose containers of vaccines have contained the preservative thimerosal, which is metabolized or degraded to ethylmercury and thiosalicylate. Thimerosal has been removed from, or reduced to trace amounts, in all U.S. vaccines recommended for children six years of age and younger, with the exception of inactivated influenza vaccine (US FDA, Thimerosal in Vaccines, 2008).
It was not until 1954-’56, after a mass methylmercury exposure occurred among the people living along Minamata Bay in Kyushu, Japan, that mercury was widely recognized as a dangerous toxin. Victims consumed contaminated fish and shellfish. The source of mercury contamination in the bay was a chemical effluent from a manufacturing company, and the mercury was bioconcentrated by the fish and shellfish living there (Watanabe and Satoh, 1996).
Pathophysiology
Organic forms, specifically methylmercury, are the most toxic of the classes of the metal. The gastrointestinal tract absorbs more than 90% of methylmercury ingested, which then enters the bloodstream and is distributed throughout the body. Because mercury binds to sulfhydryl groups, toxicity involves multiple organ systems. Structural proteins, cell membranes and enzymes are all adversely affected.
Methylmercury severely affects the central nervous system by causing psychiatric disturbances, ataxia, visual loss, hearing loss and neuropathy. Being lipophilic, methylmercury readily crosses the blood-brain barrier.
Necrosis of the proximal tubules of the kidneys is a common toxic effect. Unexplained abnormal renal function tests combined with neuropsychiatric disorders should alert the physician to consider mercury poisoning (Tan, 2008).
Manganese
It was observed by the British academic James Couper in 1837 that manganese dust produces a neurological syndrome with symptoms of muscle weakness, tremor, bent posture, whispered speech and excess salivation. These symptoms were similar to those of Parkinson’s disease, but this was not recognized until more recently. Manganese also produces adverse behavioral symptoms in humans, including anxiety, hallucinations, memory loss, cognitive problems and bizarre behaviors (McMillan, 1999).
Since the 1960s, the inhabitants of an Aboriginal village on Groote Eylandt, an island off the northeastern Australiancoast, have been known to suffer a highincidence of cerebellar, upper motor neuron, brain stem andoculomotor disorders. These disorders manifest as a numberof amyotrophic, wasting, ataxic, spastic and ophthalmoplegicclinical presentations, resembling Machado Joseph disease (MJD). It has been proposed that MJD is a multifactorial disease caused by an autosomal dominant inherited failure to regulate Mn/Mg metabolismin populations living in high Mn/low Mg ecosystems (Purdey, 2004).
Interestingly, Groote Eylandt is the site of a large manganese mine, and the affected village lies downwind of the mining operation. According to the Aboriginal elders of the village, Angurugu, the problem developed in the late 1960s when a mining corporation started open-cast extraction of manganese nearby, causing a fine black dust of manganese dioxide to cloak the village. As many as one in 30 people now living in the village suffer from the neurological stages of Groote Syndrome (Purdey, 2002).
Pathophysiology
Manganese toxicity produces a disorder with neurological symptoms similar to that of Parkinson’s disease. The central nervous system, with emphasis on the basal ganglia, is a significant target in manganese neurotoxicity.
Evidence suggests that astrocytes are an early site of damage; chronic exposure to manganese leads to dopaminergic dysfunction, neuronal loss and scarring in basal ganglia structures. There are also typical astrocytic changes known as Alzheimer Type II astrocytosis. Astrocytes have a specialized high affinity, high capacity transport system for manganese uptake, leading to high concentrations in mitochondria and disruptions of oxidative phosphorylation.
Manganese also causes other functional changes in astrocytes, including impairment of glutamate transport; alterations of glyceraldehyde-3-phosphate dehydrogenase; production of nitric oxide; and increased binding site density for the peripheral-type benzodiazepine receptor. These receptors are mainly found on the mitochondria of astrocytes, and influence oxidative metabolism, neurosteroid synthesis, and mitochondrial growth and division. The changes lead to decreased energy production, increased production of reactive oxygen species and increased concentration of extracellular glutamate. The result is abnormal astrocyte interactions with neurons, leading to the signs and symptoms of manganese neurotoxicity (Normandin and Hazell, 2002).
Case Study 1: Lead Toxicity
A 56-year-old female presented with severe vertigo and dizziness of four months duration. Many days required total bed rest, as she could not stand without assistance. She had been to see several internists and a neurologist but all of their findings and testing were non-contributory for finding the cause of the vertigo.
No neuropathies were present. An environmental toxin exposure history was taken, and it was found that she lived downwind of an automotive lead-acid battery recycling plant. She also had seven mercury amalgam dental fillings. A complete blood count (CBC) and comprehensive metabolic panel (CMP) showed no significant abnormalities; normal WBC count, liver enzymes, BUN and creatinine qualified her as a candidate for an IV EDTA provocative challenge test.
Three grams of calcium disodium EDTA were infused over 1-1/2 hours, and urine was collected for six hours and sent to the lab for analysis.
The urine toxic metals (UTM) test showed lead at 110µg/g creatinine (reference <5) and mercury at 45µg/g creatinine (reference <3). The patient had her amalgam fillings removed by a biological dentist over two clinic sessions; each of the dental treatments was followed by a 20g vitamin C infusion on the same day.
I initiated IV EDTA chelation at a frequency of two a week for one month followed by once weekly infusions for three months. A remineralization IV was administered after every five EDTA treatments; CBC and CMP were repeated; and UTM was retested after 10 and 20 chelation treatments. Symptom perturbation was noted after each of the first six chelation treatments; the most effective way to deal with this was found to be fresh cilantro consumed several times a day. Starting with the seventh chelation, the patient noted a steady linear decrease in symptoms.
The UTM collected on the day of the tenth chelation showed lead at 47µg/g creatinine and mercury at 11µg/g creatinine. The patient estimated that symptoms decreased by 70%. The UTM collected after 20 chelation IVs showed lead at 21µcg/g creatinine and mercury below detection limit of the test. The patient was experiencing mild symptoms once or twice a week. At this point, the patient was put on a comprehensive oral DMSA program at a dose of 10mg/kg, cycling three days on the drug and four days off. The oral chelation program was terminated after eight weeks, as the patient was experiencing no symptoms and felt well.
Case Study 2: Mercury Toxicity
A 50-year-old male presented desiring non-pharmaceutical treatment for hypertension. His symptoms included fatigue (4/10 on an energy scale), memory difficulties, decreased reading ability (his vision is corrected with glasses but he has difficulty making sense of what he reads), occasional numbness and tingling in feet, and cold extremities. He is irritable and gets angry without provocation, but his relationships with family and co-workers are good as he quickly recognizes that the anger is inappropriate and jokes about it.
PE shows blood pressure at 144/96mmHg with no other significant findings. Oral examination revealed 15 mercury amalgam fillings. The possibility that toxic metals may be causing his complaints was discussed and he agreed to an IV DMPS provocative challenge test. His CBC was WNL. A CMP showed a marginally increased BUN of 30mg/dL (reference 7-27mg/dL) attributed to a high protein diet; his creatinine was normal at 1.0mg/dL. For the challenge test, an IV infusion containing 20g vitamin C was established and 250mg DMPS was given by slow push through the IV line. A six-hour urine sample was collected and submitted for analysis.
The UTM test returned a mercury level of 65µg/g creatinine (reference <3) and lead of 7.9µg/g creatinine (reference <5). The patient contacted a dentist in Canada to remove his amalgam fillings and replace them with composite fillings. Upon his return, the patient was started on an IV EDTA protocol identical to Case Study 1, with the exception of a constant frequency of once weekly. His EDTA dose was set at 3g. He was also advised to take an oral chelation agent on the day of IV chelation and for two days after (3 caps TID).
After ten chelation treatments, a second DMPS challenge was administered, and his mercury was measured at 17µg/g creatinine and his lead was 3.1µg/g creatinine. Blood pressure was averaging in the range of 130/80mmHg and his presenting symptoms were decreased by an estimated 40%; he stated he was feeling better.
Ten more EDTA chelation treatments were given followed by another DMPS challenge. The UTM showed 8µg/g creatinine mercury and 3µg/g creatinine lead. His blood pressure was now measuring 120/80 on numerous occasions and his symptoms were 80% better. His extremities had become noticeably warmer, numbness and tingling were no longer present, and he was much less angry. He was put on an oral supplement program consisting of a multivitamin/mineral, magnesium citrate malate, fish oil and sustained-release alpha lipoic acid (300mg BID) to aid continued removal of mercury from the brain.
Case Study 3: Manganese Toxicity
A 47-year-old female began developing severe vertigo three months prior to presenting for naturopathic care mid-2006. She developed petit mal seizures and her legs would give way without warning. She was seen at a hospital where an EEG was performed; it was found to be abnormal and she was prescribed anti-seizure medications. She was referred to Mayo Clinic in Minnesota where, after extensive testing, they found no identifiable cause. The patient researched her symptoms online and suggested to her doctor that manganese may be a problem. He consented to order a blood manganese level that returned manganese toxicity with levels of 7.5ng/mL (reference 0.4-0.85ng/mL). Three weeks’ absence from home saw the manganese level at 3.4ng/mL and she was able to walk and read. The patient was told to seek a practitioner who could treat her closer to where she lived.
One week after returning home, the symptoms increased markedly, especially after working in the yard with a chemically treated lawn. Environmental testing of her water supply, home and work location could not identify a source of manganese. It was already known that her blood manganese was high, so a provocative challenge test was performed; 250mg DMPS was administered IV and urine was collected for six hours and submitted for UTM testing. Mercury was 19µg/g creatinine and lead was 6.9 µg/g. It was determined that IV EDTA would be the most effective chelator to address the manganese toxicity.
Four IV EDTA infusions containing 3g calcium disodium EDTA were administered, two per week, followed by a mineral replacement IV. Thereafter, EDTA and mineral replacement infusions were alternated until the patient had received a total of eight chelation treatments.
Blood manganese at the completion of chelation was 2.1ng/mL and the patient was feeling significantly better, enough so that she could return to work part-time. The patient purchased a home sauna and was given instructions for sauna detoxification. She was also put on a home detoxification program consisting of coffee enemas three to four times weekly, abdominal castor oil packs daily, and a daily homeopathic-spagyric medication basic detox kit. She had muscle pain that was addressed with 150mg magnesium citrate malate, 3-6 caps QD with meals PRN.
The patient returned to the clinic on Aug. 11 with increasing symptoms of multiple chemical sensitivity (MCS). Routine blood work and blood manganese levels were ordered. It is hypothesized that the manganese toxicity suffered two years ago has impaired the patient’s detoxification pathways, leading to an MCS presentation. A test that evaluates SNPs associated with increased risk of impaired detoxification capacity, especially when exposed to environmental toxins, was suggested to the patient.
Discussion
Readers may question why EDTA was the drug of choice for the chelation of three very different toxic metals. The safety profile of calcium disodium EDTA (CaEDTA) is favorable, and true allergies to EDTA are rare (Cranton, 2001). Adverse reactions to the EDTA infusion can usually be attributed to one of several factors:
- Infusion ingredients other than EDTA
- Preservatives added to multi-dose vials of other ingredients
- Too fast infusion rates, which can result in adverse reactions from the EDTA affecting blood mineral levels.
Lead poisoning is an FDA-approved application of CaEDTA, so it was an obvious first choice for Case Study 1.
DMPS is normally used for the IV chelation of mercury, and could have been used for Case Study 2, as the principle toxic mineral was mercury. When CaEDTA is used in a mercury toxic patient, it will bind ionic mercury in the bloodstream, but will not bind mercury attached to cell membranes as well as a dithiol chelator such as DMPS. It has been noted clinically that mercury excretion increases after the source of mercury exposure has been removed. In this case, the main exposure source was mercury amalgams.
Mercury levels on this patient were assessed with DMPS provocative challenge tests, which showed a steady decrease over time with CaEDTA chelation. This patient also had hypertension and it was felt that EDTA could help his cardiovascular condition by removing toxic metals from the artery walls. Toxic metals interfere with nitric oxide synthase (Mittal et al., 1995) and increase oxidative damage of the arteries, contributing to atherosclerosis and hypertension (Ercal et al., 2001).
CaEDTA was chosen for Case Study 3 because it is effective in removing excess manganese from tissues (Crossgrove and Zheng, 2000; Missy et al., 2000). While EDTA chelation showed usefulness in reducing symptoms, it does not remove manganese from the brain very effectively. Two years after initial treatment, the patient returned with neurological symptoms that are most likely due to manganese effects on the brain. The use of para amino salicylic acid (PAS) is being investigated as a method of removing manganese from the brain. PAS is an old tuberculosis drug that has been found to be effective in removing manganese from the brain of a patient with manganese-induced Parkinson’s disease symptoms (XXX, 2006). PAS has an adverse reaction profile similar to other salicylates, namely gastrointestinal upset and possible bleeding, platelet aggregation inhibition and renal damage. Once the PAS has been studied more completely, it may be a candidate for this patient, as she is experiencing tremors, confusion and other difficulties similar to Parkinson’s disease.
Dan Carter, ND, graduated from NCNM in 1994 and completed a two-year family practice residency at the college. He was appointed to a full-time faculty position in 1997 and set up the teaching clinic’s IV therapy clinical practicum. He also established an environmental medicine program in the school’s clinic. Dr. Carter is an instructor for IV nutritional therapy for physicians seminars, teaching since 1991. Dr. Carter is also certified by ACAM to administer chelation therapy. He has been in private practice in Bozeman, Mont., since 2004. His practice, Alpine Physicians Health Center, focuses on the nutritional treatment of many common diseases, IV nutrient therapies and chelation. Neuroendocrinology is another focal point, balancing hormones and neurotransmitters to help heal mood disorders such as depression and anxiety. He also provides support for patients undergoing cancer treatments.
References:
Ziegler EE and Filer LJ (eds): Present Knowledge in Nutrition (7th ed). Washington, D.C., 1996, ILSI Press, 336-337.
Needleman HL. History of lead poisoning in the world. National Referral Centre for Lead Poisoning in India Web site. http://www.leadpoison.net/general/history.htm. August 9, 1999.
Marcus S. Lead toxicity. emedicine from WebMD Web site. http://www.emedicine.com/EMERG/topic293.htm.
Lead poisoning. State of Washington Department of Ecology Web site. http://www.ecy.wa.gov/programs/hwtr/demodebris/pages2/lpoison.html.
Sloane J. Mercury: element of the ancients. Center for Environmental Health Sciences at Dartmouth Web site. http://www.dartmouth.edu/~toxmetal/TXSHhg.shtml. Updated March 30, 2005.
Qin Shihuang. Chinaculture.org Web site. http://www.chinaculture.org/gb/en_aboutchina/2003-09/24/content_22854.htm. September 24, 2003.
Mercury. Chem Info Net Web site. http://www.cheminfonet.org/mercury.htm.
Questions and answers on dental amalgam. U.S. Food and Drug Administration Web site. http://www.fda.gov/cdrh/consumer/amalgams.html. Updated June 3, 2008.
Hiscott G. Amalgam ban causes a stir. Dentistry.co.uk Web site. http://www.dentistry.co.uk/news/news_detail.php?id=992. February 14, 2008.
Thimerosal in vaccines. U.S. Food and Drug Administration Web site. http://www.fda.gov/cber/vaccine/thimerosal.htm. Updated June 3, 2008.
Watanabe C and Satoh H: Evolution of our understanding of methylmercury as a health threat, Environ Health Perspect 104(Suppl 2):367-379, 1996.
Tan DK. Mercury Toxicity. eMedicine from WebMD Web site. http://www.emedicine.com/ped/byname/Toxicity–Mercury.htm. Updated July 21, 2008.
McMillan DE: A brief history of the neurobehavioral toxicity of manganese: some unanswered questions, Neurotoxicology Apr-Jun;20(2-3):499-507, 1999.
Purdey M: The pathogenesis of Machado Joseph disease: a high manganese/low magnesium initiated CAG expansion mutation in susceptible genotypes?, J Am Coll Nutr 23(6), 715S-729S, 2004.
Purdey M: Dying to know the truth. Ecologist Web site. http://www.theecologist.org/pages/archive_detail.asp?content_id=394. January 11, 2002.
Normandin L and Hazell A: Manganese neurotoxicity: an update of pathophysiologic mechanisms, Metab Brain Dis 17(4):375-387, 2002.
Tuberculosis drug PAS may cure Parkinson’s like illness, Journal of Occupational Environmental Medicine June 7, 2006, 15:37.
Cranton EM (ed): A Textbook on EDTA Chelation Therapy (2nd ed). Charlottesville, 2001, Hampton Roads Publishing, 416.
Mittal CK et al: Interaction of heavy metal toxicants with brain constitutive nitric oxide synthase, Molecular and Cellular Biochemistry 149(1);August:263-265, 1995.
Ercal N et al: Toxic metals and oxidative stress part 1: mechanisms involved in metal induced oxidative damage, Current Topics in Medicinal Chemistry 1:529-539, 2001.
Crossgrove J and Zheng W: Manganese toxicity upon overexposure, NMR Biomed 17:544-553, 2004.
Missy P et al: In vitro and in vivo studies on chelation of manganese, Human & Experimental Toxicology 19(8):448-456, 2000.
XXXXXXXX: Tuberculosis drug PAS may cure Parkinson’s like illness, Journal of Occupational Environmental Medicine; June 7: 15:37, 2006.