Phranq D. Tamburri, NMD
This article explores two main areas in treating prostate disease. First, the current situation regarding prostate cancer assessment and diagnosis is discussed briefly. Liability concerns for the ND, including those now seen with andropause treatments, are mentioned. Second, qualitative and quantitative assessment tools available to NDs are described. This includes an explanation of the benefits in utilizing transrectal ultrasound of the prostate (TRUSP) imaging for patients.
The Current Situation
Prostate cancer can be a tricky pathology for the integrative physician to assess. Over the past 25 years, a prostate-specific antigen (PSA) blood test result greater than 4.0ng/ml, along with a digital rectal exam (DRE), was enough to determine one’s probable risk of having prostate cancer (CaP). A biopsy was performed as a final confirmation. This scenario has changed drastically over recent years, with the overall acknowledgement from the medical community that PSA and DRE are not reliable enough to warrant CaP treatment. Therefore, allopathic treatments will not occur (nor be reimbursed) without a prostate (TRUSP guided) biopsy. With malpractice issues ever growing, physicians became increasingly nervous about missing a CaP diagnosis once their main tool, PSA, was relatively moot. This has currently translated into a much higher incidence of biopsies recommended and performed on patients. At the same time, more patients are seeking less-invasive treatment and testing options.
Biopsy Concerns
Many naturally minded patients dread biopsy due to pain, prostatitis relapse and heavy antibiotic use following the procedure. On top of these concerns, the needle biopsy may miss a CaP altogether and create a false negative that will often be rebiopsied at a later time until a positive is “found.” In addition, many patients (and physicians) are concerned about CaP “seeding,” or the nosocomial spreading of cancer cells through the gland as the biopsy needle is pulled out. Seeding is not a concern for allopaths, understandably, because if the biopsy is negative nothing is spread. At the same time, if it is positive, any seeding is irrelevant since it is assumed the patient will have the prostate removed quickly. If the patient prefers active non-invasive treatments, however, then the biopsy potentially could have made the first problem worse and, hence, even harder to treat. These patients, therefore, are seeking alternatives to not only potential CaP treatments but also to the assessment itself.
 For NDs, this situation has created some issues. Since the only legal diagnosis for CaP is from biopsy, how do you chart a highly suspicious CaP without the procedure? … Especially when the patient refuses biopsy? What do you treat? How do you defend yourself medically if you support the patient’s right to oppose the reflex-ordered TRUSP biopsy? Generally, if a biopsy was performed and found positive, then the ND has a straight CaP diagnosis. If not, then I will diagnose the patient with an elevated total PSA (tPSA) suspicious for neoplasm of the prostate (assuming the tPSA is elevated). This is often in addition to other diagnosed pathologies, like BPH. I always state to the patient that biopsy is the only official way to diagnose CaP. I will then chart in detail the patient’s objection to biopsy before I move further into the case.
For NDs, this situation has created some issues. Since the only legal diagnosis for CaP is from biopsy, how do you chart a highly suspicious CaP without the procedure? … Especially when the patient refuses biopsy? What do you treat? How do you defend yourself medically if you support the patient’s right to oppose the reflex-ordered TRUSP biopsy? Generally, if a biopsy was performed and found positive, then the ND has a straight CaP diagnosis. If not, then I will diagnose the patient with an elevated total PSA (tPSA) suspicious for neoplasm of the prostate (assuming the tPSA is elevated). This is often in addition to other diagnosed pathologies, like BPH. I always state to the patient that biopsy is the only official way to diagnose CaP. I will then chart in detail the patient’s objection to biopsy before I move further into the case.
The importance of proper CaP assessment enters two other areas for the ND. First is the patient population that has been diagnosed with CaP on biopsy. These are patients who prefer natural treatments instead of surgery. In this case, how does the physician confidently track the cancer growth and progress of the selected therapies? Relying simply on PSA levels may not be enough.
The other unique assessment challenge is a recent issue confronting many NDs today. This is in determining CaP risk in andropausal patients before treatments. Andropause is becoming a large business for some physicians, as Baby Boomer men are now being “sold” youth in the form of botox, pharmaceuticals to treat erectile dysfunction, etc. The No. 1 andropause treatment tends to be testosterone. The standard and accepted literature at this time is very clear regarding the risk of testosterone on CaP. Testosterone does not cause CaP, but it will contribute to its progression, once established. Therefore, a push for testosterone in older (and higher CaP-risk) men has created a serious need to rule out CaP before treatment. However, since individual PSA results are questionable by themselves, how do NDs rule out such risks without a urologist ordering a reflex biopsy?
Assessment of CaP Risk and Disease Tracking
Realize that each of the following assessment tools are widely accepted and used to some degree by standard urologists. They are not used, however, to the extent explained below. This is not because urologists disagree with their cumulative validity, but due to the time and expense to add up and congregate this information. Urologists (and allopaths at large) are expected to spend ever less time on a patient case due to insurance concerns. Therefore, it is quick, efficient and financially covered to simply send for a quantitative biopsy. If a physician prefers to qualitatively determine risk without biopsy, it takes more time. This is the role an ND can successfully fill.
- Basic Risks Are they overweight? Do they have high stress markers (stress hormones or personality indicators)? Is their diet high in carbohydrates and animal protein? Are heavy metals a factor? These are risks that should be basic to the treating physician regarding all forms of cancer. Following are some specific risks for CaP: Is the patient of African-American heritage? Does CaP exist in their immediate family? A grandfather or father with CaP significantly increases a patient’s risk of developing CaP; however, it is higher still if found in a brother.
- American Urological Association (AUA) Symptom Score This is a seven-question, quantitative, one-page assessment I give every prostate patient. It scores urologic symptoms between 0 and 35. Most urologist offices and urology medical texts use this symptom score as a quick yet consistent method for tracking symptoms. It is also very useful to demonstrate whether a patient has improved under care, especially for number-minded male patients. The AUA is very important for ruling out CaP. I like to see high numbers (and symptoms), since this points to a more likely pathology of prostatitis or benign prostatic hypertrophy (BPH). CaP can also block urine outflow and give a high AUA value, but normally only when it is very pronounced. Such CaP would typically be found in patients who have not been checked for many years and have PSA values of more than 20ng/ml or even in the hundreds.
- Total PSA (tPSA) Values Despite the growing trend to distrust this lab value, especially among alternative providers, the tPSA has been determined by the AUA to still be useful in three instances: 1) to track residual CaP post-radical prostatectomy; 2) to mark the severity of BPH; and 3) to be used as CaP assessment only when incorporated as a data point to show rate of change. This is known as the PSA velocity (PSAv). By itself, a PSA may be a guide if used in the following manner: 0.0-2.0ng/ml is relatively very low risk for CaP; 2.0-10.0ng/ml requires further workup; 10.0ng/ml+ is very likely CaP or a substantial BPH. Please note that this analysis has shifted the “normal” tPSA down to 2.0ng/ml rather than the common 4.0ng/ml.
- PSAv tPSA values should not increase more than 0.75ng/ml/year. If they do, then a positive velocity exists and CaP is suspect. If the rate is greater than 2.0ng/ml/year, this suggests an aggressive pathology. A negative velocity can track positive progress regarding treatment strategies.
- Percent Free PSA (%fPSA) While the tPSA is a quantitative value, the %fPSA is a qualitative one that indicates the percent chance that the tPSA value collected is coming from a CaP or other etiology (BPH). I use this test at initial visits for a baseline and to help track treatment progression.
- PSA Density (PSAD) After determining the prostate size/volume on TRUSP (described later), you may divide the tPSA by the volume in cubic centimeters. Reference tables exist to determine if the proper (expected) PSA is being secreted per prostate cell. Higher-density cells (excessive PSA secreting) suggest an increased metabolism that is associated with CaP.
- Gleason Score If the patient had a previous positive biopsy result, this information becomes useful nonetheless in determining CaP aggressiveness. Remember, a value lower than seven is preferred (more differentiation/less aggressive) rather than one above seven (less differentiation/more aggressive).
TRUSP and Power Color Doppler Imaging
Until now I have referenced the term TRUSP with biopsy, because a diagnostic ultrasound examination of the prostate is needed to quickly guide the biopsy needle to each quadrant the urologist wishes to sample. However, for the integrative doctor, the TRUSP procedure can be used without the goal of biopsy. This allows instead for a non-invasive, qualitative image assessment of the prostate. A detailed TRUSP/Color Doppler analysis and subsequent qualitative report are then generated to determine each patient’s specific CaP risk. Although most radiation centers can provide a similar TRUSP image without biopsy if asked, they are rarely conducted by a physician, explained in detail nor interpreted by a urologist/specialist for a CaP diagnosis/assessment. This is again due to insurance reimbursement only being provided for biopsy. The centers nationwide that do implement this procedure are few but slowly growing. Nevertheless, TRUSP with Color Doppler has become an incredible tool to quickly visualize patients’ progress. I have found great success incorporating this procedure in our CaP practice. (See the accompanying illustrations for examples.)
- Volume Using TRUSP, an accurate volume measurement can be taken of the prostate gland in cc’s. This is accomplished by three measurements of the gland (X, Y, Z axis) multiplied together with a volume coefficient of 0.523. This value allows calculation of patients’ PSAD as well as gives greater meaning to the AUA symptom score.
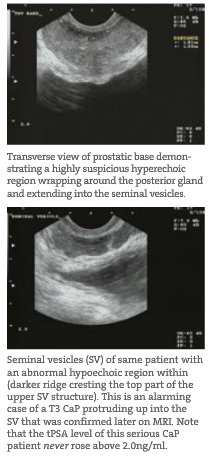
- Suspicious Lesions Suspicious areas suspect for CaP are identified, mapped and measured to the millimeter. Suspicious areas (likely cancer) are measured not only to determine current risk but for a baseline to compare treatment progress at a future time. These suspect areas are of varying density, often hyperechoic (darker compared to surrounding tissue), contain irregular borders and often reside in areas of high risk within the gland. These high-risk areas tend to be the bilateral posterolateral corners of the mid gland (compared to the base and apex regions) because the main arterial flow into the prostate is located here.
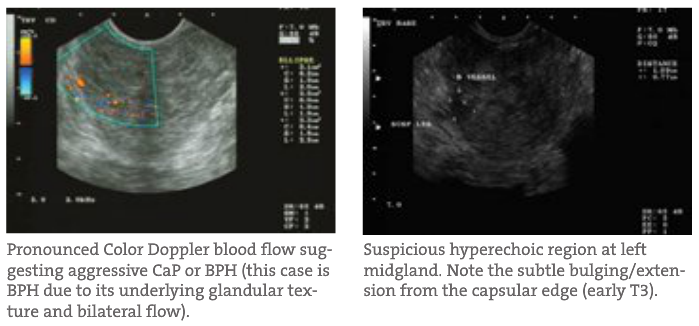
- Location Risk A CaP nestled deep within the gland is less of a current threat to metastasis than one that is either at the capsular edge or bulging outwards. The latter would constitute a potential T3 tumor (extends through the prostate capsule) and substantially higher risk to the patient. Capsular involvement is important to detect, since the PSA and other measurements may suggest a less aggressive cancer but its location may drastically alter the timeframe for treatment.
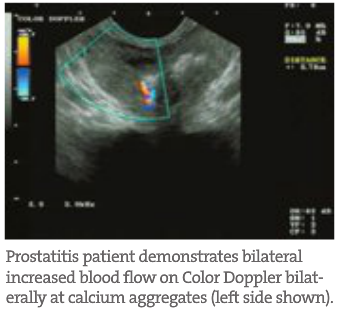
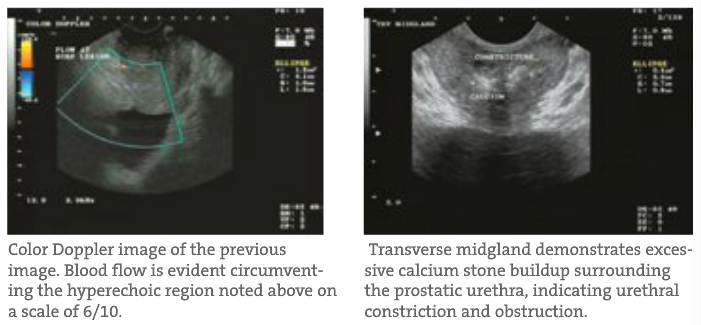
- Identification of Other Prostate Pathology BPH and prostatitis are fairly easily identified on TRUSP. BPH visualizes with large prostatic mounds often bilaterally and symmetrically with many blood vessels within each lobe. These regions also predictably demonstrate increased symmetrical blood flow on Color Doppler (explained next). Urinary retention in the bladder can also easily be identified in more progressive BPH cases. Prostatitis can be inferred from increased blood flow in tandem with large prostatic stones. Prostate stones are similar to a urolithiasis but do not flow out. Instead, they are imbedded within the gland and continually aggravate the surrounding tissue. Sometimes a positive DRE is actually picking up large calcium aggregates that a TRUSP can rule out.
- Blood Flow Often, when using a diagnostic ultrasound, the most critical of qualitative assessment tools is the Power Color Doppler. With this capability, the physician can qualitatively track and visualize blood flow quantity, velocity and location throughout the gland in real time. This helps determine patterns often seen in BPH or prostatitis compared to CaP. The latter patterns are often unilateral, asymmetric and reside around or at hyperechoic regions identified on TRUSP. BPH and prostatitis have different identifying patterns. In addition, the flow rates can determine possible CaP aggressiveness and progress when qualitatively tracked. This glandular blood flow analysis is very important when tracking patients’ healing and improvement during treatment.
CaP is difficult for any doctor to diagnose and assess without resorting to (ongoing) biopsies. The qualitative assessment for prostate pathology is perfectly suited for the ND knowledgeable in this field, since it utilizes true integration of allopathic and naturopathic principles. It also emphasizes the education of the patient during the assessment process so that he is completely aware of his individual CaP risk. Overall, your patient becomes empowered, which is the first step toward true healing.
Phranq Tamburri, NMD graduated from SCNM in 2001. He has since earned recognition as an expert in the field of prostate cancer assessment, diagnosis and treatment utilizing a balanced natural and allopathic perspective. His urology experience was earned as SCNM chief resident under Tom Kruzel, ND, and through ongoing rotations with Mayo-trained urologist Bernard Gburek, MD at Kapner and Associates, Scottsdale North Hospital. Currently, Dr. Tamburri sits as the only ND Board Member on the AZ State Supported Southwest Prostate Cancer Awareness Council. He is also head professor of clinical urology at SCNM, and is a contributing author to the upcoming Foundations of Naturopathic Medicine textbook. He practices with an international patient clientele at Naturopathic Family Care in Phoenix.