Pertussis: Resurgence with No Easy Solution
Corinne M. De Soto, ND, MS
Naturopathic Perspective
Bordetella pertussis, the bacterium responsible for causing pertussis infection and illness, has received much research and media attention lately, as rates of infection resurge.1 While some blame lower vaccine rates and others blame decreased efficacy of the acellular vaccine (Diphtheria, Tetanus and Pertussis, or DTaP) as compared to the whole-cell vaccine (DTwP), the true story is much more complicated. This article will discuss the history of vaccine development and the many factors contributing to the observed resurgence. There is wide recognition that the most effective solution would be a new vaccine that confers better protection. Naturopathic physicians can also help contribute to awareness of the problem and help promote a focus in research on other modalities that can increase vaccine efficacy, identification of mild disease, and support of those who contract pertussis illness.
Background
The DTwP vaccines were first developed in the 1930s and, along with improved hygiene, helped decrease the incidence of pertussis disease by 157-fold by 1970.2 The DTaP vaccines were developed and first used in 1997 in response to a high rate of adverse reactions to the DTwP. Asymptomatic B pertussis infection is much more common than symptomatic pertussis cough illness, and it is important to distinguish between the two, as will be done in this article. There are many different toxins secreted by B pertussis, although newer baboon models of the disease show us that it is likely that only 2 toxins are responsible for the manifestation of clinical symptoms. These are pertussis toxin (PT) and another termed “cough toxin,” which is yet to be understood or even discovered.2
Clinical Presentation
Part of the difficulty in diagnosing pertussis is the variability of clinical presentation. The disease can range from mild to severe and is often confused with other respiratory illnesses. The population most at risk of severe disease and death are infants, especially those under 6 months. Infants present with a non-distinctive, mild prodromal phase that may include coryza, sneezing, throat clearing, and mild cough. An international roundtable in 2011 determined several issues with the clinical definitions of pertussis and developed a useful algorithm (Figure 1).3
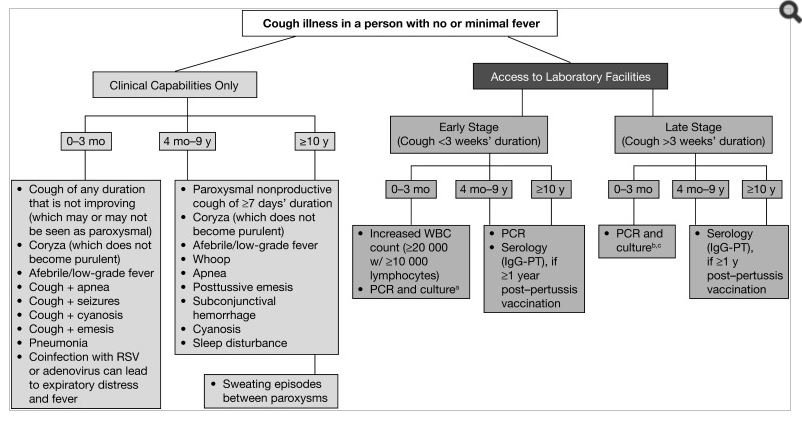
Abbreviations: IgG = immunoglobulin G; PCR = polymerase chain reaction; PT = pertussis toxin; RSV = respiratory syncytial virus; WBC = white blood cell aIn resource-limited areas where PCR is not available, samples may be sent to a reference laboratory for culture confirmation. bFalse-negatives possible. cSerology not useful in this age cohort. Diagram courtesy of Cherry JD, et al.3 Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
In infants, the Paroxysmal stage will include gagging, gasping, eye bulging, bradycardia, cyanosis, and vomiting. Infants will also have a characteristic leukocytosis with lymphocytosis that is not seen in older children and adults.2 The presentation in adults is much milder. The cough is non-productive and without wheezing. It is worse at night, with a need to sleep propped-up and with episodes of sweating. WBC, ESR, and CRP are all normal, with no or minimal fever, and the patient appears normal between coughing episodes.2,4
Decreased Efficacy of Newer Vaccines
The bacterium B pertussis was first identified as the causative agent of pertussis disease in 1903. Hygiene and other societal changes contributed to a large decline in the incidence of pertussis in the pre-vaccine era (prior to 1950). In the United States, the mortality rate per million births in the first year of life declined by about 70% over the first half of the 20th century (3530 in 1900-1919 vs 1040 in 1934-1949.5 The DTwP vaccine was widely used in the United States starting in the 1950s; from that time until the late 1990s the infant mortality rate from pertussis decreased to 2.4 per million (95% CI, 1.95-2.92).6 Pertussis incidence increased to more than 32 000 cases by 2014 (100 per million).7
The 1997 introduction of the DTaP as a replacement to the DTwP, amid concerns of a high risk of adverse events, has led to less effective protection against pertussis illness for various reasons. It has been found that immunity in both children and adults declines rapidly over a 3-year, post-vaccine time period and has minimal efficacy after that time period.8 As a result, reinfection is highly likely. In part, this seems to be due to the differing immune response between the 2 vaccines. In baboon models, it has been shown that the cellular response to the DTaP induces a Th1 and Th2 response, whereas the DTwP causes an induction of Th1 and Th17, which has a lasting and rapid recall of antibodies.9 B pertussis infection has been found to induce a pure Th17 response, which contributes to the pathogenesis of the disease, so the DTwP vaccine resembles natural infection much more so than the DTaP.9 Also, the whole-cell vaccine contains all of the pertussis antigens, which leads to a much more robust response than the DTaP, which has fewer components.10
Only in the DTaP era has evidence emerged of B pertussis mutation. PT appears to have changed from a ptxp1 to a ptxp3 form (which is more virulent), and the majority of circulating strains are now pertactin (PRN)-deficient. However, there has not yet been evidence of increased vaccine failure despite these 2 mutations, and one would need to study mild disease in order to ascertain if this is actually occurring.11
Epidemiology & Pathophysiology
Pertussis illness has a wide range of presentation, from the very severe and life-threatening to the mild cough. This makes detection especially difficult, as most adults and adolescents do not seek medical care until they have been coughing for 3-4 weeks; at this point a PCR test will usually be falsely negative, and people with mild presentations often won’t seek medical care at all.12 B pertussis can and does cause illness in any age group. Previous to modern diagnostic techniques, most adult cases were misdiagnosed,4 and some cases have been misattributed to B pertussis, since antibodies to filamentous hemagglutinin (FHA) can occur with any Bordetella species, not just B pertussis.13
Antibodies to FHA, PRN, fimbriae, and agglutinogens have previously been attributed in research to B pertussis but are now recognized as occurring with any other Bordetella spp and other non-Bordetella microorganisms. PT antibody is the only pertussis antibody associated with only B pertussis. Testing with only PT reveals that approximately 13% of prolonged coughs are due to B pertussis.14 Different pertussis toxins are attributed to specific symptoms. PT is responsible for leukocytosis with lymphocytosis and is responsible for death in infants.2 Leukocytosis with lymphocytosis will never again occur in a reinfection (in a person who previously had pertussis) or in a person who has been vaccinated, and it is never seen in adults.14 The un-named cough toxin is responsible for the paroxysmal cough and is seen in all individuals with pertussis illness, immunized and unimmunized, and both primary and repeat infections.2
Theories Regarding Resurgence
There are many theories regarding the resurgence of pertussis illness, although none alone completely explains the phenomenon. Increased sensitivity of detection via newer PCR tests contributes to this, but a 2014 analysis by the World Health Organization (WHO) has determined that the resurgence is real and not artifactual.15-17
Mutation of B pertussis to stop expressing combinations of Pertactin, FHA, and PT may play a role; however, a study in Sweden shows that a PT-only vaccine retained the same efficacy over the last 20 years despite detection of a circulating PT-absent strain.18
A newer and more plausible theory points to asymptomatic B pertussis transmission following the switch from the wP to the aP vaccine and suggests that the aP vaccine blocks symptomatic disease but not asymptomatic transmission.19 Asymptomatic disease may cause bias in favor of vaccine efficacy, but immunized individuals are likely transmitting asymptomatic disease to others.19
Strategies for Prevention
Immunization has long been the preferred method for mass prevention. Amid speculations of decreased DTaP efficacy, the question remains as to whether this is still an effective strategy. Infant immunization appears to still produce acceptable (although not ideal) protection. An observational study, conducted in 2013 by the WHO, showed that among infants aged 6-23 months in the United States, 1 and 2-dose vaccine efficacy was 50.5% (95% CI: -71.1-86.3) and 80.1% (95% CI: 41.3-93.2), respectively. Newer immunization programs for pregnant mothers also seems to be an effective strategy, as shown by a study in the United Kingdom which followed the infants of pregnant mothers, who had been given a single dose of TDaP, and their matched controls. Only 7 mothers of the 30 infants who developed pertussis at age 8 weeks or less had been immunized, as compared with 39 mothers of 55 pertussis-free control infants. Safety data for over 20 000 vaccinated pregnant women showed no increased risks of stillbirth, early delivery, maternal or neonatal death, caesarean delivery, or low birth weight, and no increased risks of severe complications that can occur in late pregnancy, such as preeclampsia, hemorrhage, fetal distress, uterine rupture, placenta or vasa previa.16 No study has looked at long-term impacts of vaccination on the health of the infant past 8 weeks.16
Another strategy, called cocooning, where all caregivers and family members of an infant are immunized, has delivered mixed results. An unpublished study in Chile, which compared cocooning with no measures, showed an 84% reduction of infant mortality in the first 6 months, although there was no decrease in the overall number cases.16 In contrast, recent models examining the incidence of asymptomatic pertussis suggest that cocooning may not be as effective as initially thought,19 and a 2015 study, published in the Pediatric Infectious Disease Journal, showed that a cocooning strategy did not significantly reduce pertussis disease in a cohort of infants less than 6 months of age.20
One solution that is being examined is altering the current aP immunization schedule to include 1 primer dose of the DTwP and then follow with subsequent DTaP along the normal schedule. This has shown good promise at drastically decreasing occurrence of pertussis in infants, while also keeping DTwP adverse events lower than the previous all wP schedule.21
There are no widely-used strategies for prevention and treatment as an alternative to immunization. Breastfeeding provides good protection against many diseases but does not appear to provide any protection against pertussis illness.22 Many parents wish to give antipyretics in the event of a fever post-vaccination, but a recently published randomized controlled trial showed that giving ibuprofen to infants after DTaP immunization significantly decreased the immune response and may decrease vaccine efficacy.23 It is unknown whether giving herbal or nutrient therapies affects the immune response post-immunization.
To date, no high-quality studies have been published for naturopathic or complementary and alternative medicine (CAM) therapies for either the prevention or treatment of pertussis illness. Many practitioners advocate the use of homeopathy, vitamin C, and botanical medicine in addition to conventional standard-of-care treatment, and even the University of Maryland Medical Center lists many natural therapies on their website to use in addition to antibiotic therapy.24 The entire medical community would benefit from the development of a body of research in CAM therapies for pertussis.
Conclusion
There are many factors involved in the resurgence of pertussis in the last couple of decades. Increased diagnostic testing, B pertussis mutation, decreased long-term efficacy of the newer aP vaccine, and its subsequent role in increasing asymptomatic infections are all likely players in this big public health concern. Both providers and patients would benefit from the development of a newer, effective vaccine, a change to the current vaccination schedule, and research looking at the efficacy of natural therapies in the enhancement of the immune response to immunizations as well as treatment and prevention of B pertussis infection and disease.
References:
- Science Daily. Whooping cough resurgence due to vaccinated people not knowing they’re infectious? June 24, 2015. Science Daily Web site. https://www.sciencedaily.com/releases/2015/06/150624071018.htm. Accessed May 10, 2017.
- Cherry JD. The History of Pertussis (Whooping Cough); 1906–2015: Facts, Myths, and Misconceptions. Curr Epidemiol Rep. 2015;2(2):120-130. Available at: https://link.springer.com/article/10.1007/s40471-015-0041-9. Accessed May 10, 2017.
- Cherry JD, Tan T, Wirsing von Konig CH, et al. Clinical definitions of pertussis: summary of a Global Pertussis Initiative roundtable meeting, February 2011. Clin Infect Dis. 2012;54(12):1756-1764.
- Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18(2):326-382.
- Mortimer EA Jr, Jones PK. An evaluation of pertussis vaccine. Rev Infect Dis. 1979;1(6):927-934
- Chow K, Khandaker G, McIntyre P. Global Childhood Deaths From Pertussis: A Historical Review. Clin Infect Dis. 2016;63(Suppl 4):S134-S141.
- Centers for Disease Control and Prevention. Pertussis (Whooping Cough): Surveillance and Reporting. Last updated January 10, 2017. CDC Web site. http://www.cdc.gov/pertussis/surv-reporting.html. Accessed May 30, 2017.
- Koepke R, Eickhoff JC, Ayele RA, et al. Estimating the effectiveness of tetanus-diphtheria-acellular pertussis vaccine (Tdap) for preventing pertussis: evidence of rapidly waning immunity and difference in effectiveness by Tdap brand. J Infect Dis. 2014;210(6):942-953.
- Warfel JM, Zimmerman LI, Merkel TJ. Comparison of three whole-cell pertussis vaccines in the baboon model of Pertussis. Clin Vaccine Immunol. 2015;23(1):47-54.
- Cherry JD. Comparative efficacy of acellular pertussis vaccines: an analysis of recent trials. Pediatr Infect Dis J. 1997;16(4 Suppl):S90-S96.
- Pawloski LC, Queenan AM, Cassiday PK, et al. Prevalence and molecular characterization of pertactin-deficient Bordetella pertussis in the United States. Clin Vaccine Immunol. 2014;21:119-125.
- Mink CM, Cherry JD, Christenson P, et al. A search for Bordetella pertussis infection in university students. Clin Infect Dis. 1992;14(2):464-471.
- Prince HE, Lieberman JM, Cherry JD. Age-related differences in patterns of increased Bordetella pertussis antibodies. Clin Vaccine Immunol. 2012;19(4):545-550.
- Cherry JD. The epidemiology of pertussis: a comparison of the epidemiology of the disease pertussis with the epidemiology of Bordetella pertussis infection. Pediatrics. 2005;115(5):1422-1427.
- Jackson DW, Rohani P. Perplexities of pertussis: recent global epidemiological trends and their potential causes. Epidemiol Infect. 2014;142(4):672-684.
- World Health Organization. WHO SAGE Pertussis working group. Background paper. SAGE April 2014. March 14, 2014. Available at: http://www.who.int/immunization/sage/meetings/2014/april/1_Pertussis_background_FINAL4_web.pdf. Accessed May 15, 2017.
- Lapidot R, Gill CJ. The Pertussis resurgence: putting together the pieces of the puzzle. Trop Dis Travel Med Vaccines. 2016; 2:26. Available at: https://tdtmvjournal.biomedcentral.com/articles/10.1186/s40794-016-0043-8. Accessed May 10, 2017.
- Martin SW, Pawloski L, Williams M, et al. Pertactin-negative Bordetella pertussis strains: evidence for a possible selective advantage. Clin Infect Dis. 2015;60(2):223-227.
- Althouse BM, Scarpino SV. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med. 2015;13:146.
- Healy CM, Rench MA, Wootton SH, Castagnini LA. Evaluation of the impact of a pertussis cocooning program on infant pertussis infection. Pediatr Infect Dis J. 2015;34(1):22-26.
- DeAngelis H, Scarpino SV, Fitzpatrick MC, et al. Epidemiological and Economic Effects of Priming With the Whole-Cell Bordetella pertussis Vaccine. JAMA Pediatr. 2016;170(5):459-465.
- Pandolfi E, Gesualdo F, Carloni E, et al. Does Breastfeeding Protect Young Infants From Pertussis? Case-control Study and Immunologic Evaluation. Pediatr Infect Dis J. 2017;36(3):e48-e53.
- Wysocki J, Center KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35(15):1926-1935.
- University of Maryland Medical Center. Pertussis. Last reviewed April 27, 2016. UMM Web site. Available at: http://www.umm.edu/health/medical/altmed/condition/pertussis. Accessed May 30, 2017.
Image Copyright: <a href=’https://www.123rf.com/profile_dolgachov’>dolgachov / 123RF Stock Photo</a>
 Corinne M. De Soto, ND, MS, is an NUNM graduate with a dual degree – a Doctorate of Naturopathic Medicine and a Masters of Science in Integrative Medicine Research. Dr De Soto is the owner and medical director of The Koa Clinic of Integrative Healing in Kailua Kona, HI, and is the first naturopathic physician on the Big Island of Hawaii (and second in the state) to be credentialed as an in-network PCP with HMSA, Hawaii’s Blue Cross Blue Shield affiliate. She is also the co-host of the annual Hawaii Doc Talks Conference, a mother of two young girls, and a coffee farmer.
Corinne M. De Soto, ND, MS, is an NUNM graduate with a dual degree – a Doctorate of Naturopathic Medicine and a Masters of Science in Integrative Medicine Research. Dr De Soto is the owner and medical director of The Koa Clinic of Integrative Healing in Kailua Kona, HI, and is the first naturopathic physician on the Big Island of Hawaii (and second in the state) to be credentialed as an in-network PCP with HMSA, Hawaii’s Blue Cross Blue Shield affiliate. She is also the co-host of the annual Hawaii Doc Talks Conference, a mother of two young girls, and a coffee farmer.