Beat the Heat: Non-Hormonal Approaches to Hot Flashes and Night Sweats
Bradley Bush, ND
Approximately one-third of women in the United States are approaching or have gone through menopause.1 Although menopause is a natural transition stage in a woman’s life, 75% of women (roughly 40 to 50 million) will experience menopausal vasomotor symptoms (VMS), such as hot flashes and night sweats, and 60% will seek medical treatment for them.1
The Women’s Health Initiative (WHI), a 2002-published study of hormone therapy in postmenopausal women,2 has created increased demand for non-hormonal approaches to the alleviation of VMS in menopause. Naturopathic doctors have traditionally used botanicals, herbs, dietary and lifestyle treatment approaches. Since the WHI, there has been a growing understanding of the mechanisms behind VMS, which has enabled the integration of additional neuro-endocrine approaches to menopausal symptom relief.
A Neuro-Endocrine Mechanism
There is no clear consensus on the pathophysiology of VMS in menopause; however, a number of theories exist. The premise behind most proposed mechanisms is a narrowing of the thermoregulatory threshold between shivering and sweating, caused by changing estrogen and neurotransmitters levels.3 Key players include central norepinephrine, estrogen, serotonin and endorphins.
Low estrogen levels are the hallmark of menopause. This results in decreased circulating levels of serotonin and endorphins, as well as fewer serotonin receptors. A drop in serotonin receptors causes a loss of norepinephrine negative feedback and a reduction in the thermoregulatory set point. This premise explains how therapies that increase serotonin, estrogen, and endorphin levels, or that lower norepinephrine levels, can often reduce VMS in menopause.
Central Norepinephrine
VMS is triggered in a small region of the hypothalamus, and central noradrenergic activity plays a major role in its initiation.4 The α2-adrenergic agonist, clonidine, which blocks central norepinephrine activity, has been shown to reduce hot flashes.5
Conversely, α2-adrenergic antagonists, like yohimbine, increase central norepinephrine levels, leading to more hot flashes.5 Increased central norepinephrine activity narrows the thermoregulatory threshold, worsening VMS, while lower central norepinephrine activity expands the threshold and improves VMS. Reducing central norepinephrine activity in the brain, especially within the hypothalamus, is the main proposed mechanism behind the effectiveness of selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), gamma-aminobutryic acid (GABA) agonists, and endorphin promoters in the amelioration of hot flashes and night sweats.
Estrogen-Serotonin
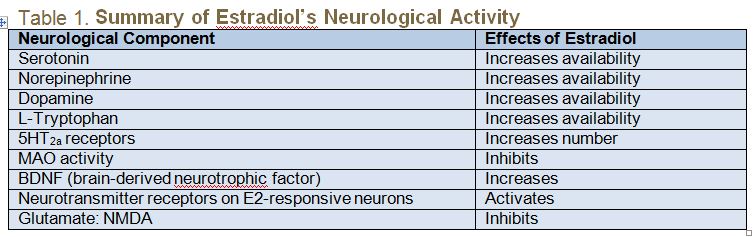
Estrogen and serotonin receptors coexist throughout the body. Many of the perceived benefits of estrogen may actually be the result of estrogen’s effects on serotonin (5-hydroxytryptamine, or 5HT). (See Table 1 for a summary of estradiol’s neurological activity.) Estradiol impacts serotonin in 3 ways:
- It increases tryptophan hydroxylase, the rate-limiting enzyme in serotonin synthesis;
- It inhibits monoamine oxidase (MAO), the enzyme responsible for degradation of monoamines such as serotonin; and
- It stimulates an increase in 5HT2a serotonin receptor sites.6 5HT2a serotonin receptor sites play a key role in the occurrence of hot flashes.7
Selective serotonin reuptake inhibitors (SSRIs) block the reabsorption (reuptake) of the neurotransmitter serotonin in the brain. Increased circulating 5HT levels result in greater 5HT2a receptor activity and reduced norepinephrine activity. This mechanism is the reason SSRIs significantly reduce menopausal VMS up to 60% of the time.8,9
Endorphins
Estradiol also enhances endorphin synthesis. Endorphins along with serotonin have been shown to modulate central norepinephrine activity.10 Endorphin synthesis is stimulated by estrogen, and lower levels of estrogen lead to lower circulating endorphins and less control over norepinephrine.11 Good healthy living can naturally increase endorphins. Examples include exercise, dancing, laughing, eating high-quality foods, and engaging in romance/sexual play.12
Prescriptive, Non- Hormonal Therapies
The thermoregulatory threshold is narrowed by reductions in serotonin and estrogen levels or increases in norepinephrine.13 A number of pharmaceutical studies.8,13,14 confirms these proposed mechanisms, providing a road map for natural, non-hormonal approaches to VMS in menopause.
Studies examining the antidepressants, paroxetine and venlafaxine, demonstrated a reduction of menopausal VMS and improved patient quality of life.14 However, these antidepressants have also been shown to have undesirable side effects, including loss of libido, insomnia, headaches, and nausea. Clonidine produced positive results in small studies, but it also induced side effects like nausea, fatigue, headaches, dizziness, and dry mouth.12 Studies on gabapentin, an anticonvulsant, have shown mixed results and side effects, including fatigue, dizziness, ataxia, and somnolence.14
Natural, Non-Hormonal Approaches
Several natural non-hormonal approaches to VMS have shown promise:
- 5-Hydroxytryptophan (5-HTP): This amino acid intermediary in the synthesis of serotonin is easily absorbed when taken orally. It readily crosses the blood-brain barrier and is converted to serotonin by the actions of the enzyme, amino acid decarboxylase. One small study (n=24) indicated that 150 mg nightly was not enough to sufficiently reduce VMS.4 Dosing of 5-HTP must be personalized to the patient’s needs.15 I typically screen the patient with a urinary serotonin screen and then dose accordingly (50-200 mg before bed). If testing is not an option, I generally start at a dose of 50 mg and titrate upward by 50 mg every 5-7 days, not to exceed 200 mg.
- 4-amino-3-phenylbutyric acid: This phenyl derivative of GABA easily crosses the blood-brain barrier, binds to GABA receptors, and may increase GABA levels.16 GABA is known to decrease central norepinephrine activity. This GABA derivative is becoming a commonly prescribed supplement by physicians to address anxiety and insomnia. It also has good anecdotal evidence for its use in reducing the intensity and severity of menopausal VMS. Dosing varies individually (higher doses are recommended if anxiety, insomnia, and/or inflammation are present), with common dosing of 300-600 mg at bedtime, and 100-300 mg in the morning, if needed.
- Black cohosh: Used for many years in the treatment of hot flashes, Cimicifuga racemosa (black cohosh) has been approved by the German Commission E for the treatment of menopausal VMS. The exact mechanism is unknown, although it is hypothesized to reduce serotonin reuptake and 5HT2a serotonin receptor activity. This is a very safe botanical and provides support to about 50% of the women using it. Dosing depends on the preparation; a dose of 20 mg BID is commonly used in research.14
- Soy Isoflavones: Soy is one of the richest sources of isoflavones, compounds which are similar to endogenous estrogen. Isoflavones compete with estrogen for the same receptors and exert both estrogenic and anti-estrogenic effects. Data does not indicate that soy isoflavones are effective as a single therapy; however, as a nutritional supplement, or through dietary means, 40-80 mg per day can be supportive for menopausal VMS. 17
Conclusion
Hot flashes and night sweats greatly affect the quality of life of many menopausal and peri-menopausal women. Declining estrogen levels spark imbalances in the endocrine and nervous systems. By understanding the mechanisms behind VMS, natural non-hormonal treatments can be personalized for patients, offering safe and highly efficacious alternatives or complements to hormone-based therapies.
References:
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes. 2005;3:47.
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321-333.
- Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23(2):117-125.
- Freedman RR. Treatment of menopausal hot flashes with 5-hydroxytryptophan. Maturitas. 2010;65(4):383-385.
- Freedman RR, Woodward S, Sabharwal SC. Alpha 2-adrenergic mechanism in menopausal hot flushes. Obstet Gynecol. 1990;76(4):573-578.
- Rybaczyk LA, Bashaw MJ, Pathak DR, et al. An overlooked connection: serotonergic mediation of estrogen-related physiology and pathology. BMC Womens Health. 2005;5:12.
- Berendsen HH. The role of serotonin in hot flushes. Maturitas. 2000;36(3):155-164.
- Albertazzi P. Noradrenergic and serotonergic modulation to treat vasomotor symptoms. J Br Menopause Soc. 2006;12(1):7-11.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomized controlled trial. Lancet. 2000;356:2059–2063.
- Tsuda K, Tsuda S, Nishio I, Masuyama Y. Effects of beta-endorphin on norepinephrine release in hypertension. J Cardiovasc Pharmacol. 2000;36 Suppl 2:S65-S67.
- Curtis AL, Leiser SC, Snyder K, Valentino RJ. Predator stress engages corticotropin-releasing factor and opioid systems to alter the operating mode of locus coeruleus norepinephrine neurons. Neuropharmacology. 2012;62(4):1737-1745.
- Hawkes, CH. Endorphins: the basis of pleasure? J Neurol Neurosurg Psychiatry. 1992; 55(4):247–250.
- Umland EM. Treatment strategies for reducing the burden of menopause-associated vasomotor symptoms. J Manag Care Pharm. 2008;14(3Suppl):14-19.
- North American Menopause Society. Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement of The North American Menopause Society. Menopause. 2007;14(2):168-182.
- Curcio JJ, Kim LS, Wollner D, Pockaj BA. The potential of 5-hydryoxytryptophan for hot flash reduction: a hypothesis. Altern Med Rev. 2005;10(3):216-221.
- Lapin I. 4-amino-3-phenylbutyric acid, Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug. CNS Drug Rev. 2001;7(4):471-481.
- Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr. 1998;68(6 Suppl):1333S-1346S.
 Bradley Bush, ND is the director of Clinician Affairs for NeuroScience, Inc, which in conjunction with Pharmasan Labs, provides licensed health care providers with integrative clinical assessments and proprietary nutraceuticals to identify and target neurological and hormonal imbalances. A graduate of the National College of Naturopathic Medicine, Dr Bush specializes in neuro-endo-immune health, nutrition, and infusion therapies. He is a co-author of the ND: Notes Science Board Review, founder and past organizer of the annual Pharmaceutical Perspectives conference, and currently serves on the board of the Naturopathic Education and Research Consortium (NERC).
Bradley Bush, ND is the director of Clinician Affairs for NeuroScience, Inc, which in conjunction with Pharmasan Labs, provides licensed health care providers with integrative clinical assessments and proprietary nutraceuticals to identify and target neurological and hormonal imbalances. A graduate of the National College of Naturopathic Medicine, Dr Bush specializes in neuro-endo-immune health, nutrition, and infusion therapies. He is a co-author of the ND: Notes Science Board Review, founder and past organizer of the annual Pharmaceutical Perspectives conference, and currently serves on the board of the Naturopathic Education and Research Consortium (NERC).